Guide Biomedical Solutions, LLC
(Guide-Bio)
Comprehensive
Tech Advisory Services
for
FDA-regulated Manufacturers
Guide-Bio is a proud member of Key
U.S. Government R&D and Manufacturing Consortia
Wrap-around services, from pursuit to post-market surveillance
Pursuit | Proposal | Grant capture
Guide-Bio is an accepted member of the BARDA BioMaP and RRPV consortia.
Since 2020, Guide-bio has helped several clients win over $85 million in combined non-dilutive funding from USG agencies.
Guide-bio has assisted clients engaged with several USG and international biotech agencies, including NIH, BARDA, ARPA-H, and CEPI.
“Made-in-USA” Supply Chain Development
Guide-Bio created a teaming arrangement, resulting in a multi-year HHS contract for the domestic manufacture of COVID-19 rapid antigen tests.
Guide-Bio has developed a coalition model, providing low-cost, mass-vaccination capability in support of BARDA Project NextGen.
Guide-bio assists TAA-compliant companies to onshore manufacturing and secure funding in support of USG medical countermeasure (MCM) initiatives.
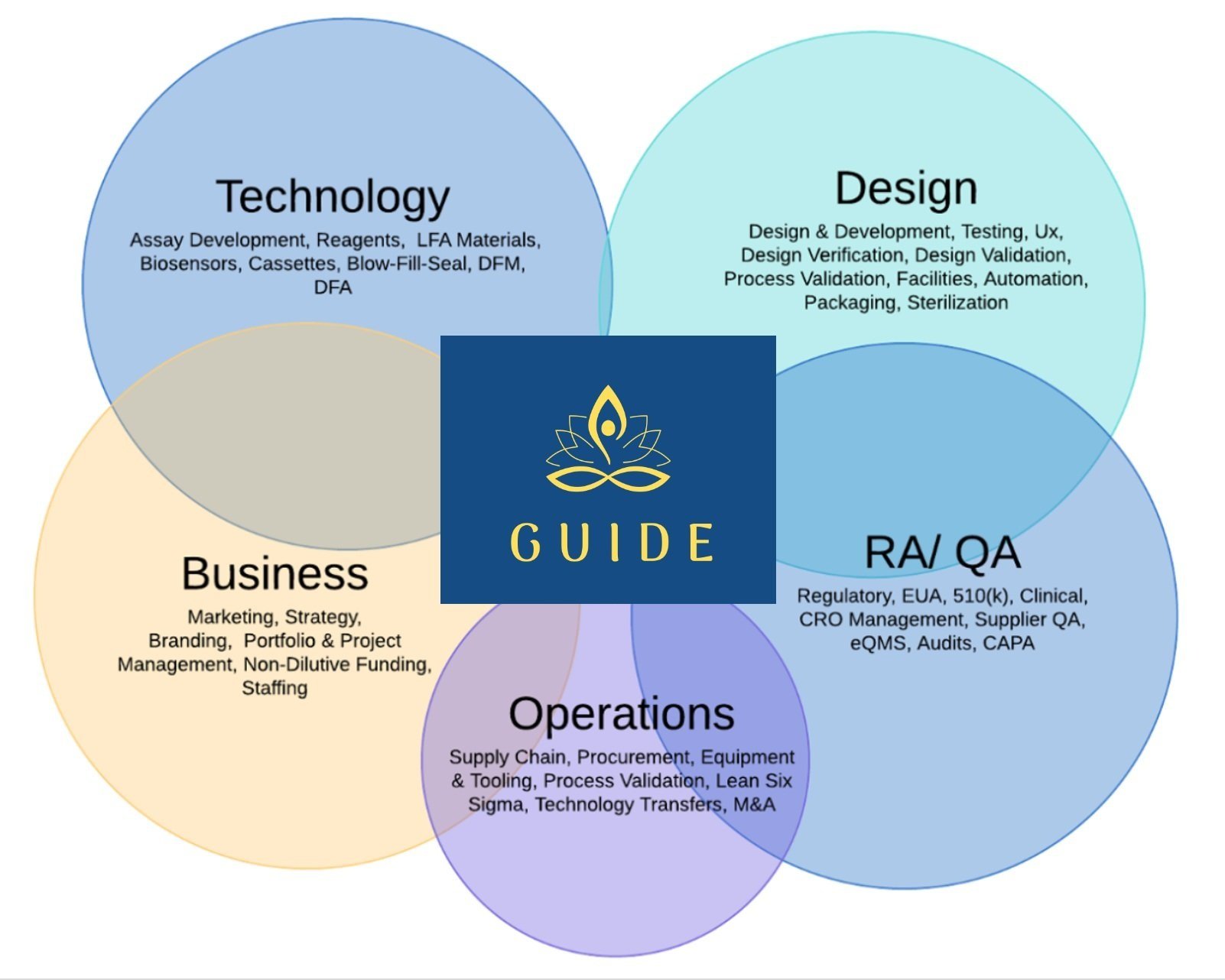
Comprehensive support across the entire product life cycle
Guide-bio provides access to over 100 life science and biotech subject matter experts, providing clients with end-to-end support across the entire product life cycle,including:
Portfolio and Project Management
Fractional C-level support (CEO, COO, CFO…)
Market research
Customer Discovery
IP landscape review
Regulatory strategy
Concept Engineering & Validation
Design & Development
CRO management
Supply chain development
Procurement
Quality Management Systems (QMS)
Cold chain logistics and distribution
Manufacturing support and scale-up
Post-market surveillance (PMS)
Get in Touch with Us
If you'd like to learn more about how Guide Biomedical Solutions may support your current or future projects, please use the contact form to arrange an introductory call or virtual meeting.
Our team will respond quickly to discuss how we may support your strategic goals.